Solar cells have great potential as a source of clean electrical energy, but so far they have not been cheap, light, and flexible enough for widespread use. Now a team of researchers led by Tandon Associate Professor André D. Taylor of the Chemical and Biomolecular Engineering Department has found an innovative and promising way to improve solar cells and make their use in many applications more likely.
Most organic solar cells use fullerenes, spherical molecules of carbon. The problem, explains Taylor, is that fullerenes are expensive and don't absorb enough light. Over the last 10 years he has made significant progress in improving organic solar cells, and he has recently focused on using non-fullerenes, which until now have been inefficient. However, he says, "the non-fullerenes are improving enough to give fullerenes a run for their money."
Think of a solar cell as a sandwich, Taylor says. The "meat" or active layer - made of electron donors and acceptors - is in the middle, absorbing sunlight and transforming it into electricity (electrons and holes), while the "bread," or outside layers, consist of electrodes that transport that electricity. His team's goal was to have the cell absorb light across as large a spectrum as possible using a variety of materials, yet at the same time allow these materials to work together well. "My group works on key parts of the 'sandwich,' such as the electron and hole transporting layers of the 'bread,' while other groups may work only on the 'meat' or interlayer materials. The question is: How do you get them to play together? The right blend of these disparate materials is extremely difficult to achieve."
Using a squaraine molecule in a new way - as a crystallizing agent - did the trick. "We added a small molecule that functions as an electron donor by itself and enhances the absorption of the active layer," Taylor explains. "By adding this small molecule, it facilitates the orientation of the donor-acceptor polymer (called PBDB-T) with the non-fullerene acceptor, ITIC, in a favorable arrangement."
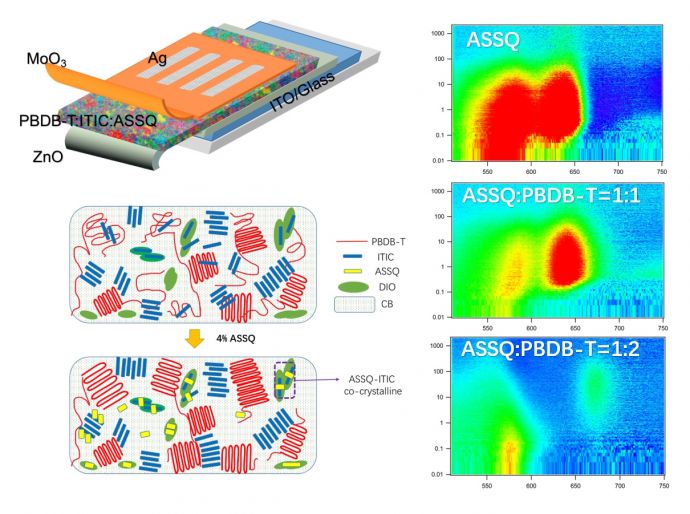
This solar architecture also uses another design mechanism that the Taylor group pioneered known as a FRET-based solar cell. FRET, or Förster resonance energy transfer, is an energy transfer mechanism first observed in photosynthesis, by which plants use sunlight. Using a new polymer and non-fullerene blend with squaraine, the team converted more than 10 percent of solar energy into power. Just a few years ago this was considered too lofty a goal for single-junction polymer solar cells. "There are now newer polymer non-fullerene systems that can perform above 13 percent, so we view our contribution as a viable strategy for improving these systems," Taylor says.
The organic solar cells developed by his team are flexible and could one day be used in applications supporting electric vehicles, wearable electronics, or backpacks to charge cell phones. Eventually, they could contribute significantly to the supply of electric power. "We expect that this crystallizing-agent method will attract attention from chemists and materials scientists affiliated with organic electronics," says Yifan Zheng, Taylor's former research student and lead author of the article about the work in the journal Materials Today.
Next for the research team? They are working on a type of solar cell called a perovskite as well as continuing to improve non-fullerene organic solar cells.
---
In addition to Taylor and Zheng, co-authors are Jiang Huang and Junsheng Yu of the University of Electronic Science and Technology of China; Gang Wang of Northwestern University; Jaemin Kong, a post-doctoral researcher now at NYU; and Di Huang, Megan Mohadjer Beromi, and Nilay Hazari of Yale. The National Natural Science Foundation of China, the Project of Science and Technology of Sichuan Province, the U.S. National Science Foundation, and the Yale Institute for Nanoscience and Quantum Engineering supported the research.
This story is courtesy of New York University Tandon School of Engineering.


